Why is Chrome and Nickel Plating Important for Corrosion Resistance?
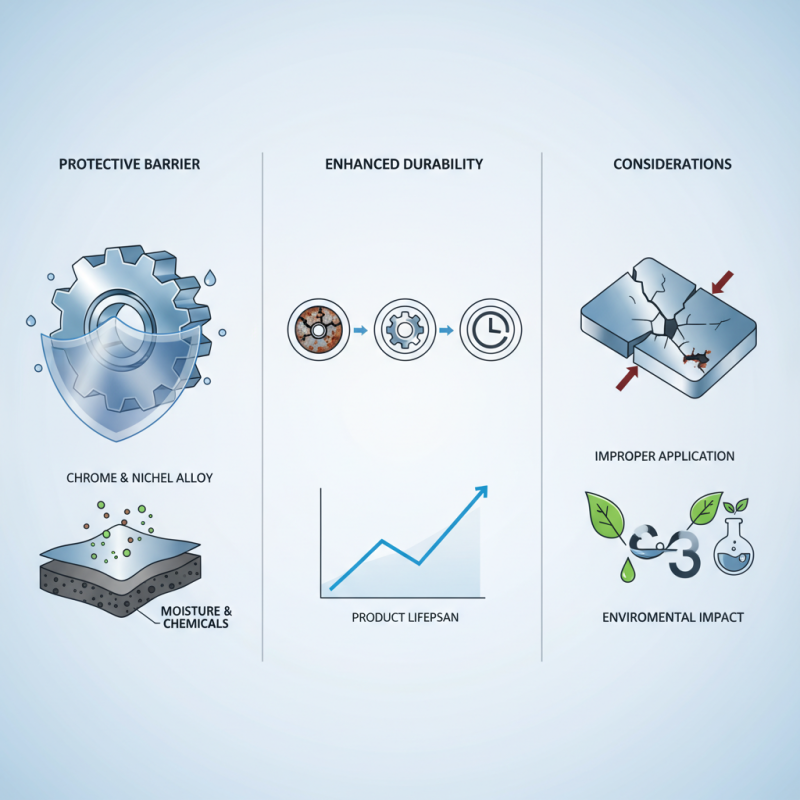
Chrome and nickel plating plays a crucial role in corrosion resistance. This process enhances the durability of various metal surfaces. Metals often face rust and wear when exposed to moisture and chemicals.
The application of chrome and nickel plating creates a protective barrier. This barrier prevents moisture from reaching the underlying metal. It also reduces the likelihood of rust formation, extending the life of products. For instance, automotive parts benefit significantly from this plating process. They maintain their appearance and functionality longer.
Despite its benefits, some issues can arise. Improper application may lead to uneven coatings. This can result in exposed areas vulnerable to corrosion. Additionally, the environmental impact of plating chemicals raises concerns. Finding a balance between effective protection and sustainability is essential.
The Role of Chrome Plating in Enhancing Corrosion Resistance
Chrome plating plays a significant role in enhancing corrosion resistance. This process involves applying a thin layer of chromium onto a metal surface. The result is a shiny, reflective finish that not only looks good but also protects the underlying material. Chrome plating forms a barrier against moisture and other corrosive agents. Without this layer, metals can rapidly degrade.
When you consider metal components that face harsh environments, chrome plating is often essential. It prevents rust and oxidation, extending the lifespan of items like automotive parts and tools. However, the quality of the plating matters. Thick or uneven coatings can develop cracks. These flaws can allow moisture in, compromising the protection. Regular inspections are necessary to ensure the integrity of the chrome layer.
Tips: To maintain chrome-plated items, clean regularly with a soft cloth. Avoid abrasive materials that could scratch the surface. If you notice signs of wear, re-plating may be needed. Don't overlook the importance of proper maintenance; it can prevent bigger issues down the line. Awareness of these factors can greatly enhance the effectiveness of chrome plating for corrosion resistance.
Understanding Nickel Plating's Benefits for Metal Protection
Nickel plating is a key process for protecting metals from corrosion. It provides a protective barrier against environmental elements. This layer helps to extend the lifespan of various metal products, especially in challenging conditions.
In industries such as automotive and aerospace, nickel plating is essential. It enhances both durability and appearance. The glossy finish can make metal parts more attractive. However, not all nickel plating processes are the same. Some may not adhere well or wear off quickly. It's crucial to choose quality techniques and materials to ensure effectiveness.
Tips:
When selecting nickel plating, consider thickness. A thicker layer often offers better protection. Pay attention to the base metal. Some metals bond better with nickel than others. Regular maintenance can also prolong the effectiveness of the plating. Always inspect for signs of wear or corrosion. This proactive approach can save costs later.
Comparison of Chrome and Nickel Plating Techniques

Chrome and nickel plating are two widely used techniques for enhancing corrosion resistance in various applications. Chrome plating involves applying a thin layer of chromium onto metal surfaces. This layer provides a shiny, aesthetically appealing finish. It is also hard, making the surface resistant to scratches and wear. However, the process can be costly and requires precise control to avoid defects.
Nickel plating is another effective technique. It offers excellent corrosion protection and can be applied in various thicknesses. Nickel is more flexible, allowing for better adhesion and uniformity. It can also enhance electrical conductivity. However, it may not provide the same level of shine as chrome. Improper application can lead to issues like peeling or uneven coating.
Both methods have their pros and cons. The choice between them often depends on the specific requirements of an application. One needs to consider factors like cost, appearance, and performance under stress. Some applications may require a combination of both for optimal results. Ultimately, both techniques play a crucial role in preventing corrosion but have unique characteristics that should be carefully evaluated.
Applications of Chrome and Nickel Plating in Various Industries
Chrome and nickel plating play vital roles in many industries. These coatings enhance the durability of materials, making them resistant to corrosion. In the automotive sector, for instance, chromed parts not only look appealing but also withstand harsh weather. They ensure a longer lifespan for vehicle parts, which is crucial for manufacturers and consumers alike.
In the aerospace industry, the importance of corrosion resistance cannot be overstated. Nickel plating is often used on crucial components like landing gears and engine parts. These components undergo extreme conditions, and any failure can lead to serious consequences. Yet, despite the rigorous standards, sometimes even plated parts fail due to hidden defects or inadequate application processes.
Furthermore, in the manufacturing sector, chrome and nickel plating are vital for tools and machinery. They reduce wear and tear, enhancing performance. However, issues can arise during the plating process, causing inconsistencies. It’s essential for industries to continually review and refine their plating techniques to avoid costly mistakes. Overall, while chrome and nickel plating offer significant benefits, attention to detail is key to ensuring their effectiveness in demanding applications.
Factors Influencing the Effectiveness of Plating for Corrosion Resistance
Chromium and nickel plating serves as a key shield against corrosion. The effectiveness of this process can be influenced by various factors. One major aspect is surface preparation. Proper cleaning and conditioning of the base metal enhance adhesion and effectiveness. If the surface is not adequately treated, the coating may peel or flake over time.
Another factor is the thickness of the plating. Thicker layers typically offer better protection. However, they may be more susceptible to cracking under stress. Balancing thickness is essential. The plating process itself also varies; electroplating and electroless plating have distinct advantages. Each method impacts corrosion resistance differently. Environmental conditions, such as humidity and temperature, play a role too.
The quality of materials used in the plating process is crucial. Impurities can weaken corrosion protection. Observing and maintaining proper plating parameters ensure both durability and effectiveness. Manufacturers must continuously refine their techniques. Achieving optimal corrosion resistance is a work in progress, filled with lessons learned.
Importance of Chrome and Nickel Plating for Corrosion Resistance
Related Posts
-

Ultimate Guide to Bright Chrome Plating Benefits Techniques and Applications
-
2025's Top Chrome Plating Techniques: Enhance Durability and Aesthetics
-
Top 10 Uses of Chromium in Electroplating You Need to Know
-

Why You Should Know the Different Types of Chrome Plating for Your Projects
-
Best 10 Benefits of Copper Chrome Plating for Industrial Applications?
-

2026 Top Trends in Decorative Metal Plating for Modern Aesthetics?
